water molecule
Water Molecule

WATER MOLECULE. No one in the factory asks where the food molecules came
Water molecules
Water Molecules. Image Credit: National Science Foundation

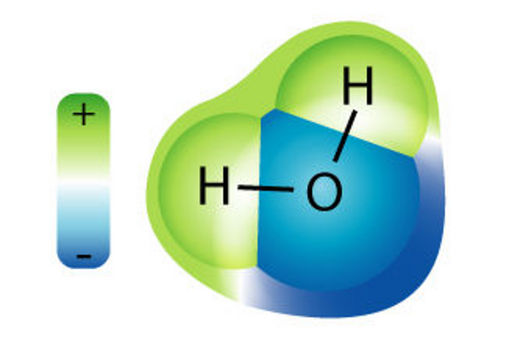
A water molecule. In this configuration the H2O acts as a polar molecule,

water molecule image on an aquatic background

Six molecules of water plus six molecules of carbon dioxide produce one

Because molecules are smaller than light waves, they cannot be observed
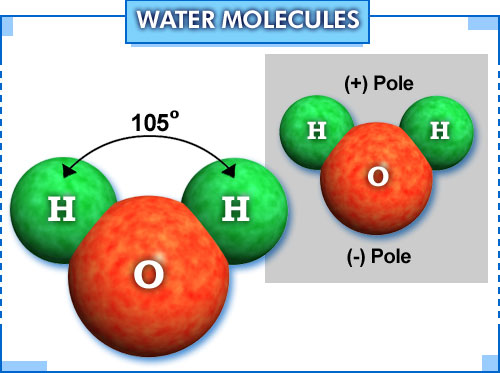
one molecule of water has two hydrogen atoms covalently bonded to a single

Water Molecule Liquid

A diagram of a water molecule, with dimensions

Water-Molecule

The H2O Molecule: What Is Water?

water molecule with oxygen emphasised on a black background

Small, Yes, but Mighty: The Molecule Called Water. Serge Bloch

Do more strongly hydrogen-bonded water molecules reorient more slowly ?

Water and Ice

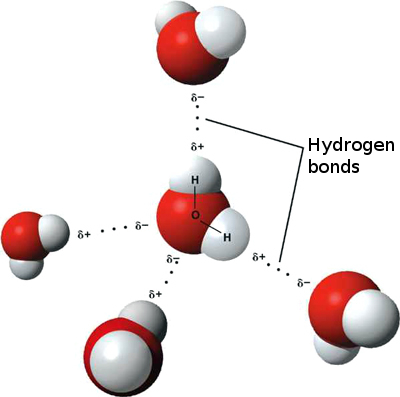
Attraction of water molecules to charged groups in marcromolecules produces

Water Molecule. Stock photo; File #: 1892614. Back to results
No comments:
Post a Comment